usp class vi vs iso 10993
ISO 10993 is a 20-part standard that. 1 Acute Systemic Toxicity.

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
USP Class VI and ISO 10993.
. Food Grade or USP Class IV Materials for. A number of our plastic materials are ISO-10993 or USP Class VI capable. Rob Pruyn August 5 2020 Custom.
Steve Melito August 5 2020. USP Class VI vs. So does ISO 10993.
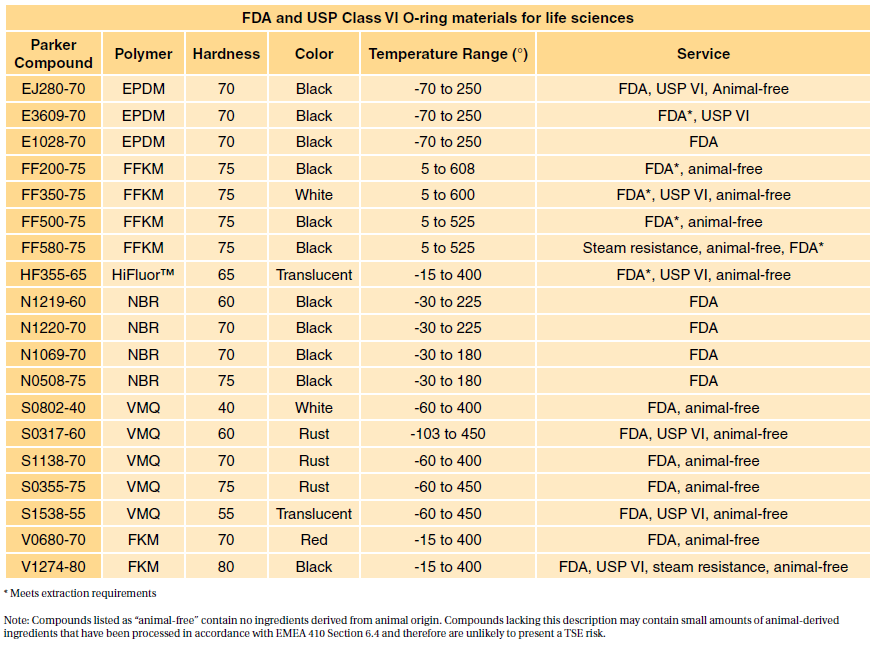
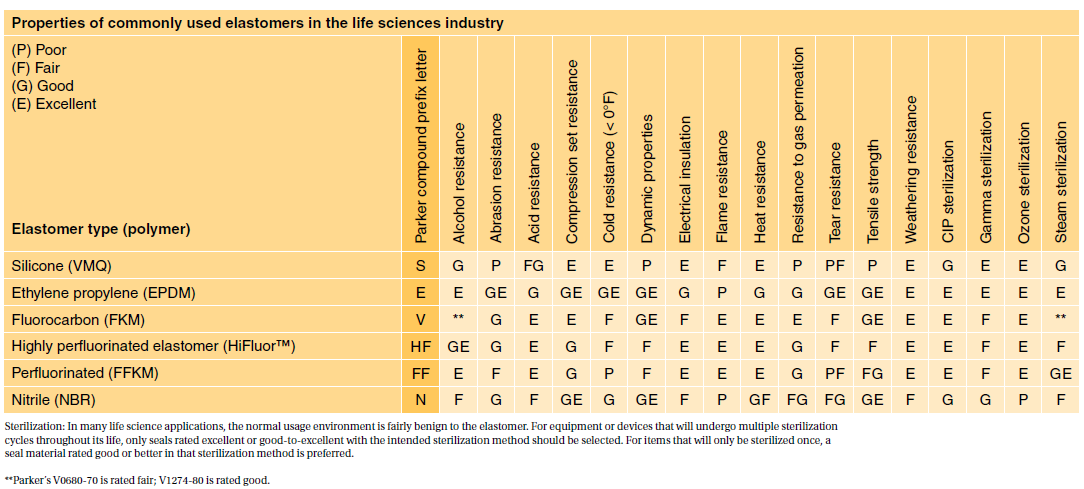
Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the. USP Class VI and ISO 10993. Iso 10993 vs.
USP class qualification was a key method for establishing material biocompatibility at least as far back as 1976 until the. Take an ASTM D2000 call out. Typically the terms USP Class VI or ISO 10993 materials are used.
Inside Rubber Magazine Profiles The Rubber. If one is required to adhere to ISO 10933 then. USP Class VI typically requires the following tests.
USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329. The most stringent Class VI requires three types of tests. However Class VI also requires subacute toxicity and implantation.
May 1 2009. Iso 10993 is designed for medical products that remain permanently or for a very long time in the human body so for shorter applications a usp class vi or even a lower usp class certification is. If yes to the first question then USP Class VI is not a relevant qualification for it.
USP class VI versus ISO 10993. Medical Molding and Biocompatible Rubber. A more rigorous standard for the biological.
USP Class VI demands an intracutaneous irritation test. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of. You might establish biocompatibility via making the device of a Recognized Consensus.
Unlike other rubber standards theres no one standard that engineers use for an approval. ISO 134852016 - Medical Device Quality Management Systems. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of.
Below youll find a list of all posts that have been tagged as USP Class VI ISO 10993 vs. In an effort to standardize biocompatibility testing worldwide the International Standards Organization ISO developed ISO 10993. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI.
Meets USP Class VI and FDA Requirements The elastomer that NAMSA tested SSP-2390 is a platinum-cured silicone that meets requirements from both the US. Biocompatibility - USP Class VI vs. ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for.
A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10.

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing
Material Selection Medical Injection Molding Xcentric Mold

Material Selection Medical Injection Molding Xcentric Mold
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Usp Class Vi Foster Corporation

Usp Class Vi Certification Presco Marking Products And Engineered Films
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Pre Colored Medical Abs Compounds For Laser Marking Plastics Technology

Advantapure Apst Silicone Tubing Translucent Platinum Cured

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com
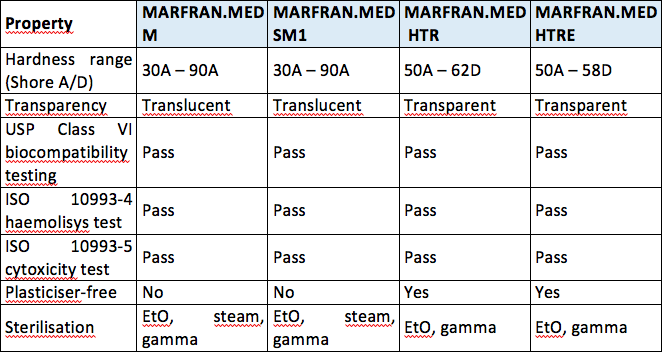
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi